Background:
A small, virtual biotech company developing a difficult-to-express bispecific fusion protein needed to advance quickly from discovery to current good manufacturing practice (CGMP) material for clinical studies. The molecule was initially expressed in Human Embryonic Kidney (HEK) cells, but the process was not scalable or regulatory –friendly. A previous attempt to switch the protein to Chinese hamster ovary (CHO) cells had failed to produce an active high-yield product.
With limited in-house CMC expertise and an aggressive 12-month timeline, the company sought a partner capable of solving complex process challenges and transitioning production from HEK to CHO while preparing for CGMP manufacturing.

Challenges:
- Expression Barrier: The bispecific fusion protein was difficult to express; previous attempts in CHO had produced low-activity material.
- Timeline Pressure: The client needed to reach CGMP production in ~12 months – much faster than a typical cell line + process development path.
- Molecule Complexity: The protein included a cleavable linker for cytokine delivery and showed pH sensitivity, creating risk of product variants (HMW/LMW species).
- Limited CMC experience: As a virtual biotech, the client relied heavily on CDMO guidance for regulatory–ready process development.
Approach:
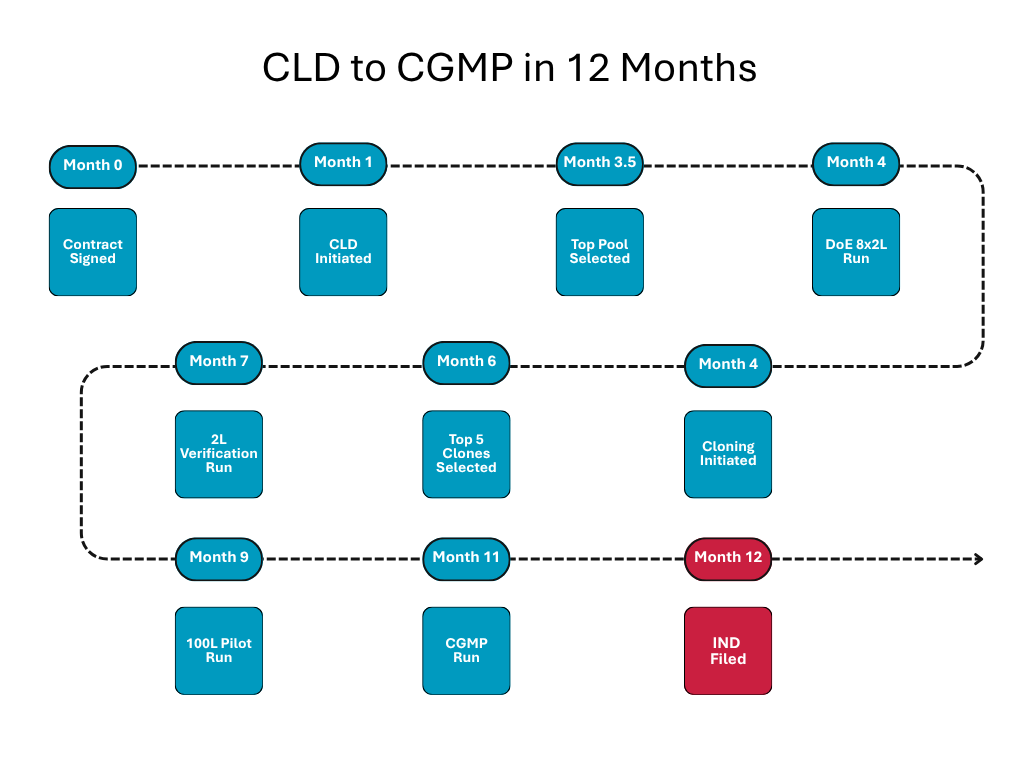
- Strategic HEK to CHO Transition: The Avid team used its cell line development (CLD) expertise to engineer a CHO-cell clone capable of 7 g/L expression – more than 10x productivity compared to the original HEK process. The agreement was milestone-based to manage technical risk: if CHO expression failed, the client could exit.
- Parallel Development to save Months: Instead of waiting the typical 6-7 months for a final clone selection, process development began at month 4 using a stable pool representative of the expected final clone. This overlapped CLD and process work, compressing the timeline.
- Risk-Based Analytical Planning: Because final material wasn’t yet available, the team applied risk-based method planning to build analytical strategies early and keep development on track.
- Downstream Process Innovation: The protein’s extreme pH sensitivity required nonstandard purification.
- Used small-scale high-throughput techniques to explore pH and chromatography conditions.
- Designed a custom mixed-mode resin workflow to protect the monomer and remove HMW/LMW species.
- Collaborative, Transparent Communication: Close technical interaction-built trust and provided the client with CMC know-how it lacked internally.
Results:
- CHO Success: Delivered a high-producing CHO cell-line (7g/L titer) where previous attempts failed.
- Accelerated Timeline: Overlap of CLD and process development cut months off the standard path, reaching CGMP-ready production within 12 months.
- Robust, Scalable Process: Custom purification strategy addressed the molecule’s pH sensitivity and ensured regulatory-quality product.
- Trusted Partnership: The client gained CMC confidence and a clear commercialization path despite limited internal expertise.
Expert Insights:
- Early Risk Mitigation Enables Speed: Starting process development in parallel with CLD – when managed by experienced teams – can compress timelines significantly.
- Tailored Purification Matters for Novel Modalities: Complex proteins often require custom chromatography and pH control beyond standard mAb platforms.
- Transparent Collaboration Build Confidence: for virtual or early-stage companies, the right CDMO can function as an extension of the internal CMC team.
- Strategic Partnering De-Risks Scale-Up: Early technical assessment and milestone-based contracts let companies pursue aggressive timelines without undue risk.
