Background:
A biotech company approached Avid Bioservices to prepare a monoclonal antibody (mAb) process for CGMP manufacturing under a compressed timeline.
The client had already developed an initial upstream and downstream process but was not designed with manufacturing facility constraints in mind. Early analysis suggested the process would require large chromatography columns, extended cycle ties, and long intermediate holds – driving up cost of goods and threatening scheduling efficiency.
To derisk the transfer and stay on timeline, the client engaged Avid to re-engineer the process using a “facility-fit” strategy aligning development with the physical and operational realities of production suites.

Challenges:
- Manufacturing constraints: original design required large columns and multiple cycles, creating long processing hours and extended hold times.
- Cost Pressure: High consumable use and long processing hours and extended intermediate hold times projected higher cost of goods than benchmark mAb processes.
- Variable upstream Feed: Ongoing upstream development meant changing titers and impurity levels, complicating downstream design
- Tight Timeline: Process needed to be ready for CGMP manufacturing in just 2 months of development work.
Approach:
- Early Facility-Fit Calculations: Performed a facility-fit calculation for the client’s process including column sizing, flow rate, buffer volume, and cycle time modeling to identify bottlenecks and cost drivers before development began.
- High-Capacity Resin Screening: Focused on higher binding-capacity resins for all three chromatography steps (protein A, AEX, CEX) to reduce column size and cycle count while maintaining purity.
- Worst-Case Process Development Strategy: Used worst-case starting material (higher impurity, variable feed) to ensure robustness despite ongoing upstream changes.
- Parallel Workstreams: Ran downstream process development concurrently with upstream optimization, saving calendar time while improving impurity removal and yield.
- Continuous Improvement & Automation: Implemented automated column efficiency testing to improve reproducibility and reduce manual labor during both PD and manufacturing
Results:
- Faster Development: Completed process development and verification in ~2 months.
- Smoother Tech Transfer: Process fit seamlessly into Avid’s manufacturing suites with no unexpected scale-up issues
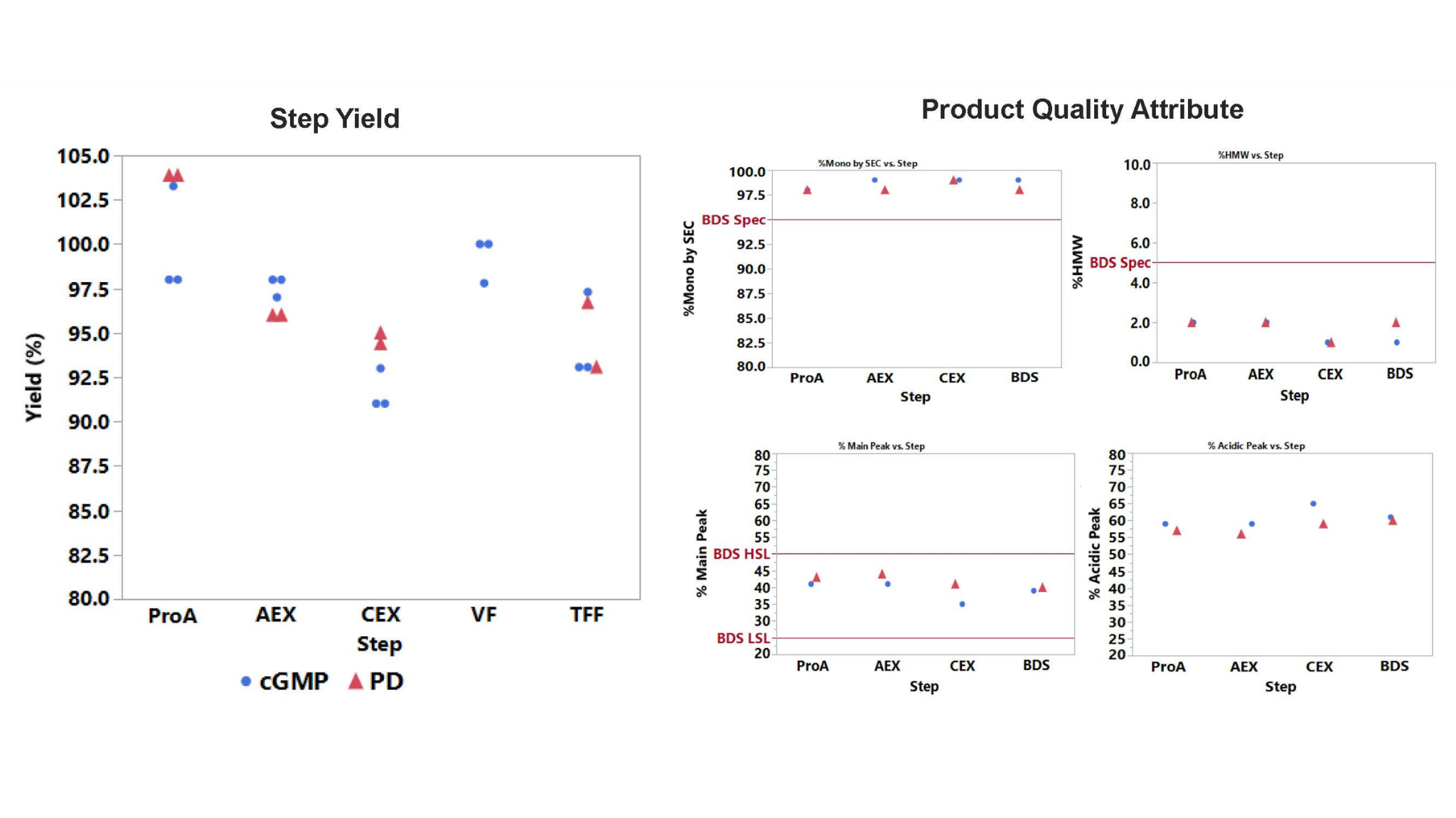
- Higher Productivity: Increased chromatography step capacities 2-3x vs. original process, reducing cycle count and processing hours by >50%
- Lower COGS: process changes reduced production costs by 25%
- Consistent Scale-Up: PD and CGMP runs mirrored each other in yield and product quality, meeting critical quality attributes (CQAs)
Expert Insights:
- Facility-Fit Planning Saves Time and Cost: Early modeling of real plant constraints avoids late redesigns and costly retrofits
- High-Capacity Resins Can Unlock Efficiency: With careful screening and elution optimization, higher loadings reduce cycles and consumables.
- Design for Worst Case Feeds: Anticipating variability from upstream development creates a more robust, future-proof process.
- Parallel Development Accelerates Timelines: Overlapping upstream and downstream work can safely compress schedules when managed by experienced teams
- Continuous Improvement Pays off: Introducing automation column efficiency testing enhances consistency, accuracy, and efficiency and reduces labor long-term.
