Customized flexible solutions for your every need.
From concept to patients, let us personally take you there.

Franklin - Drug Substance Line 1
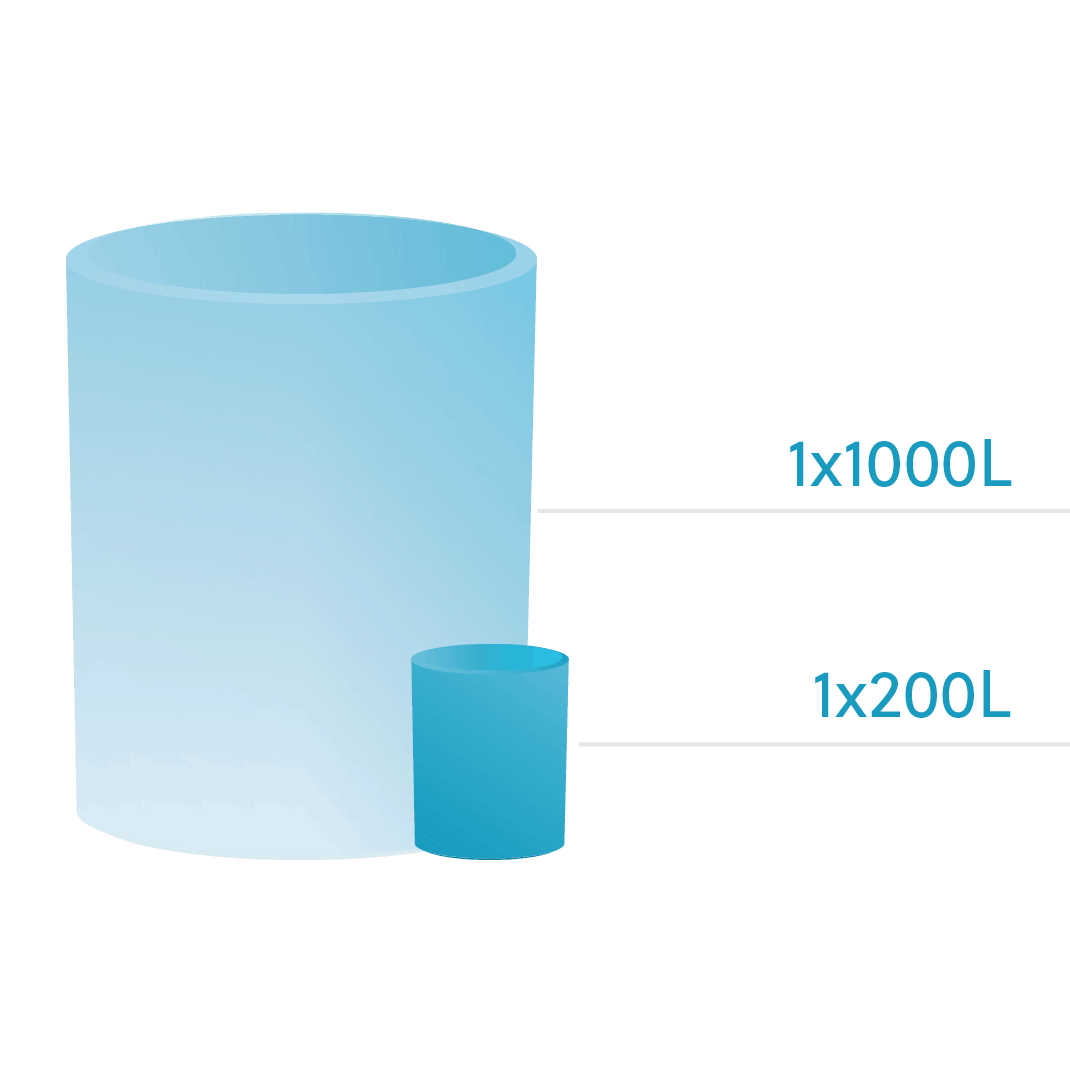
Located in Tustin, CA, our legacy facility (commissioned in 1993) and FDA licensed since 2005, offers full supporting utilities and infrastructure for early-phase and commercial projects. This facility can accommodate both stainless steel and single-use bioreactors with capacity ranging from 100L up to 1000L.
- Legacy facility commissioned 1993
- ~12,000 ft2 of cleanroom & supporting areas
- FDA licensed for commercial manufacture since 2005
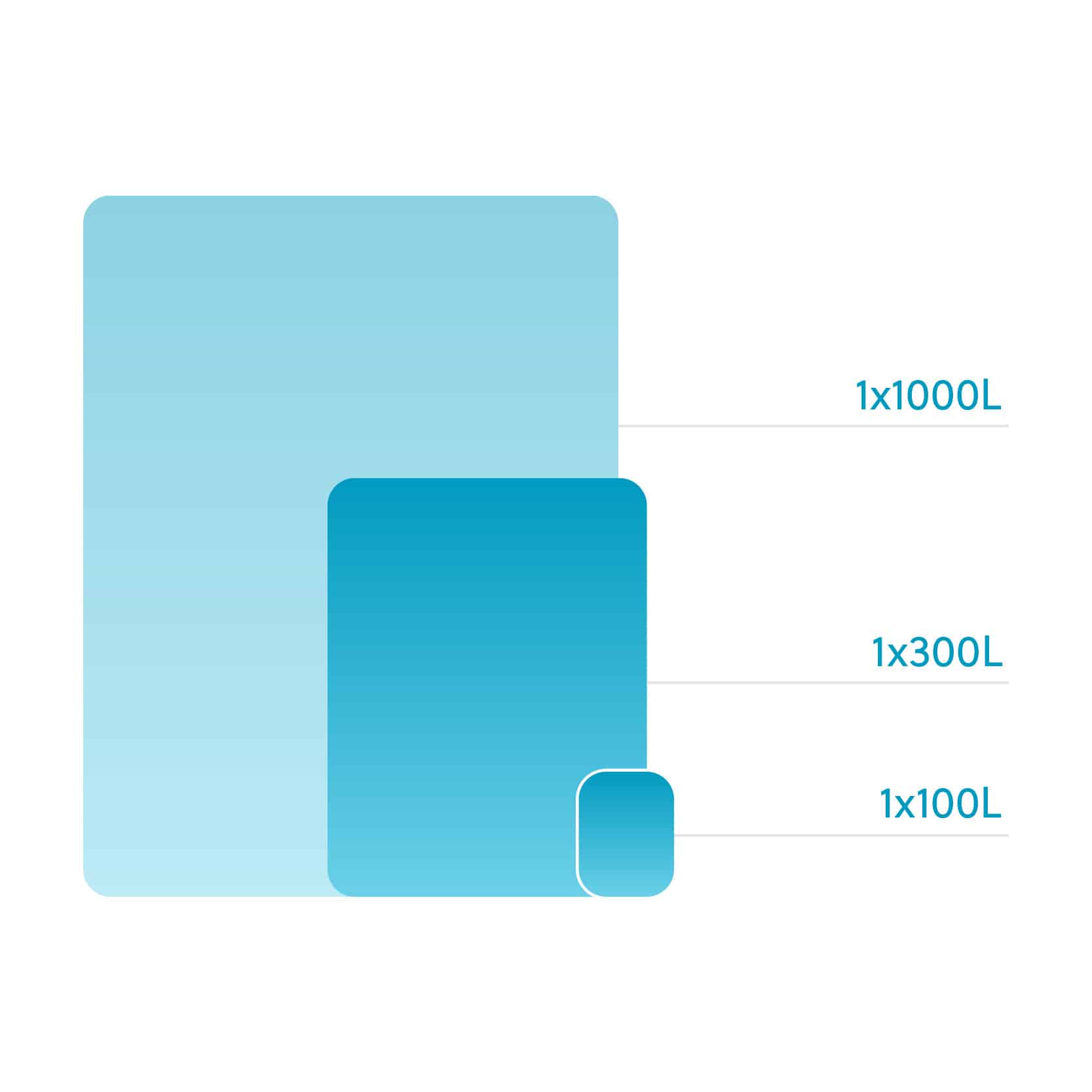
Our flexible manufacturing scales provide multiple solutions for all project types. We also have disposable components in both upstream and downstream processes. The expanding capacity of our facilities makes it one of a kind.
Our flexible contract manufacturing scales provide multiple solutions for all project types. We also have disposable components in both upstream and downstream processes. The expanding capacity of our facilities makes it one of a kind.