Thought Leadership

Article
Mar 14, 2024
A Viral Vector CDMO Vision that Resonates
Building a viral vector manufacturing facility from the ground up. Members of Avid Bio's leadership team discuss the key considerations that went into designing their viral vector manufacturing facility and how it will deliver quality viral vector process development and CGMP manufacturing.

Article
Oct 17, 2023
Cell and Gene Therapy Insights: Can AAV continue to deliver the promise of gene therapy?
Charlotte Barker (Editor, BioInsights) speaks to Elie Hanania, PhD (Vice President of Process Development Viral
Vector Technologies, Avid Bioservices) about the current status and future manufacturing of
AAV-based gene therapies, including how to streamline large-scale manufacturing.

Article
May 10, 2022
Approaching Viral Vector Manufacturing With An Emphasis On Quality & Design
Adapting an existing facility for viral vector manufacturing purposes presents challenges that have the potential to impact safety and integrity. A dedicated facility, built from the ground up, enables CDMOs to address areas of concern specific to viral vectors. Learn more about how Avid’s purpose-built approach is answering the industry need for quality viral vector manufacturing.

Article
Oct 13, 2021
A Customer-Centric Approach to Cell & Gene Therapy Manufacturing
Learn more about the drivers motivating Avid Bio’s entrance into the viral vector space, and the unique and differentiating value the company can offer cell and gene therapy developers.
Article
Nov 09, 2021
Shortening Biopharmaceutical Process Development Timelines with High-Throughput Approaches.
Jeanette Doerr, Ph.D. & Haiou Yang, Ph.D.
Article
Oct 13, 2021
A Customer-Centric Approach to Cell and Gene Therapy Manufacturing
Drew Brennan

Article
Sep 17, 2021
Expanding and Serving the Market with Flexible Single-Use Capacity
Michael Alston, Jr.

Article
Jun 29, 2021
Strong Quality Systems and Quality Culture Ensure Inspection-Ready Status
Ray Marzouk

Article
May 12, 2021
Unique Commercial Experience Primes Avid Bioservices for Growth
Nicholas Green
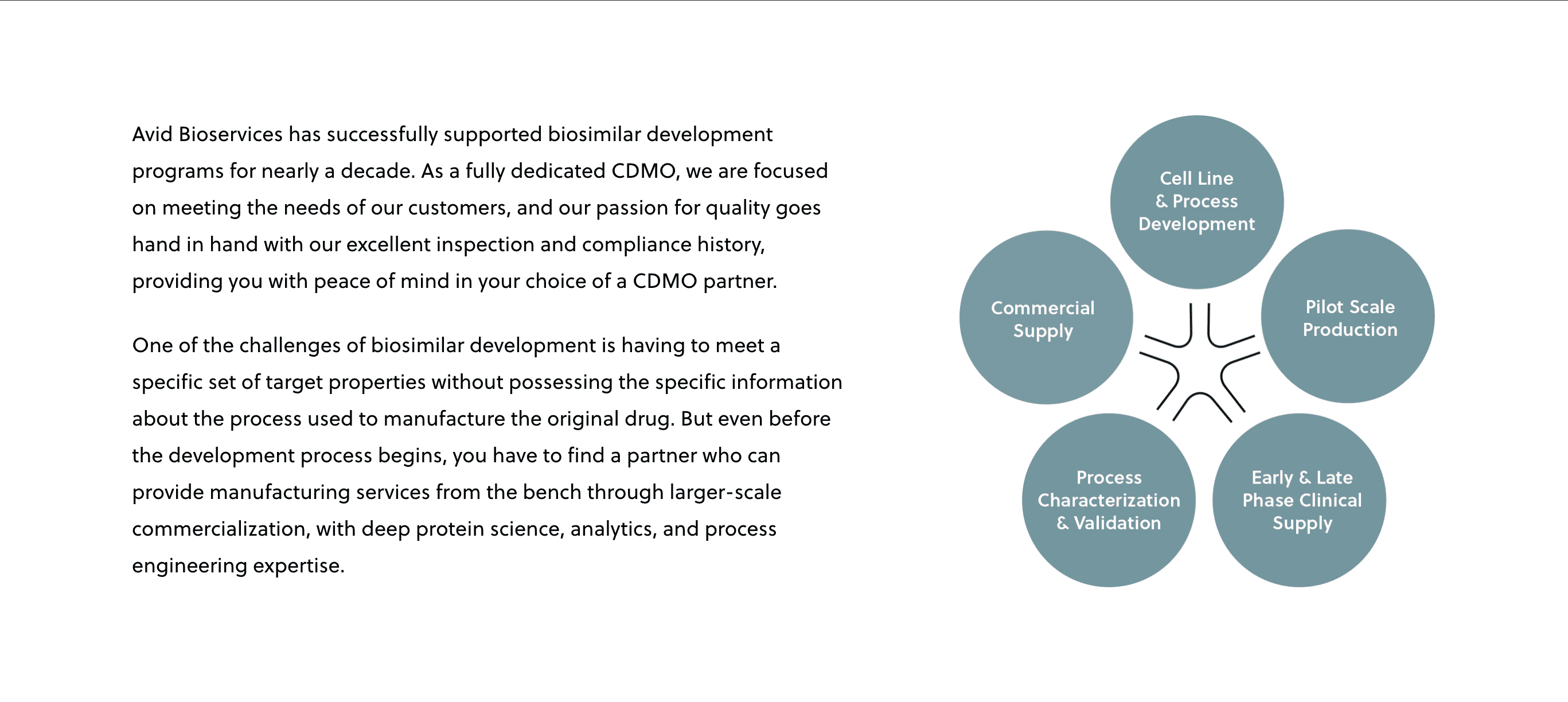
Article
Mar 18, 2021
Successful Biosimilar Development Requires an Outsourcing Partner with the Right Experience
Richard Richieri